|
Let’s do a 2021 roundup, a roundup of what we already know about MS, and what we hope to learn about this disease.
Here are the most important things we already know about MS (established facts):
Notice that MS is not identified here as an “autoimmune” disease. While many persons in the field believe that MS is an autoimmune disease, no clearcut “autoimmune antigen” has ever been identified. The current enthusiasm among scientists for myelin, for instance, as the target of the immune system in MS must be tempered by the realization that the oligoclonal bands (antibodies) in the spinal fluid of MS patients generally do not target myelin. More on this subject later . . . Here are the things we have shown in our lab, and can be relatively sure about (i.e. additional facts):
These two things – overexpressed microbial sequences and spinal fluid antibodies against some of these microbes – make us much more enthused about the idea that microbes might be causing MS. Here are some things we don’t know, but would like to find out:
Thought Experiment – The Hot Pepper Let’s think about MS in a different way, starting with a “thought experiment.” This is a hypothetical experiment that we just think about and never actually perform for reasons which will become obvious. Einstein used to do thought experiments to illustrate a point. As my family, friends, and colleagues will tell you, I am no Einstein, but let’s try this anyway. Suppose I have a hot pepper. Maybe a jalepeno or habanero, but really hot. And it doesn’t really matter if the pepper is freshly picked and still alive, dead and dried out, or crushed, the result will be the same.
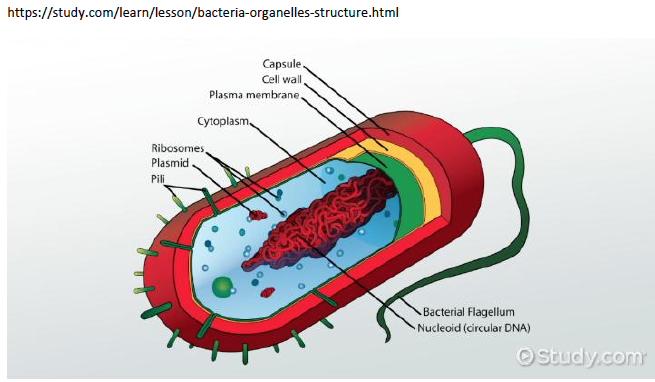
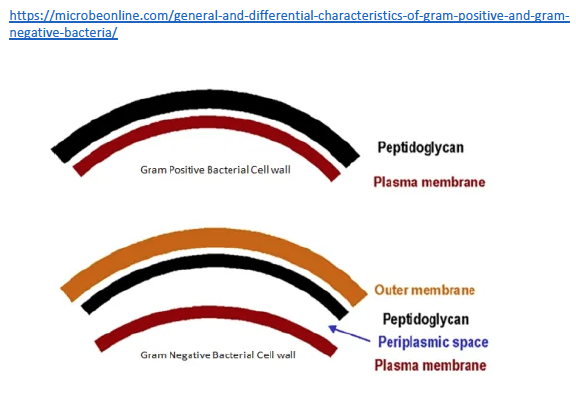
And maybe this will work if it’s small enough to be easily eaten (phagocytosed) and removed. But if it’s large enough, the macrophage removal process will not be enough, and that pepper will fester. Surrounding tissue will be damaged and destroyed, and our unfortunate patient will have chronic pain, organ dysfunction, and maybe even perforation of the adjacent bowel (not good). So how is this even remotely relevant to MS? Well, it is relevant because those microbial sequences we found in MS brain tissue are attached to their own little hot peppers. That is, they are made of microbial proteins, including one class that is really irritating – peptidoglycan. Peptidoglycans give bacterial cell walls their structure. Without them bacteria fall apart. And peptidoglycans are irritating, dead or alive. The immune system of animals and humans has evolved to detect and eradicate peptidoglycans.
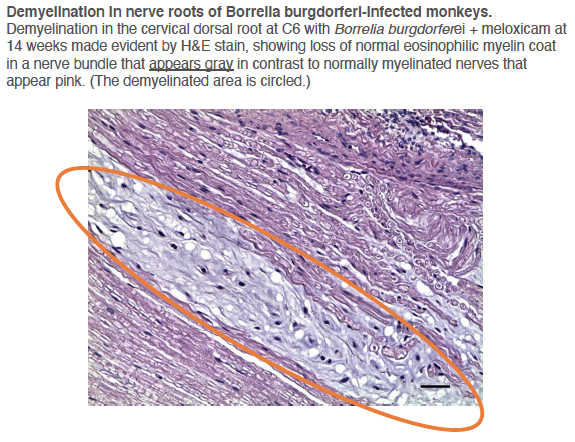
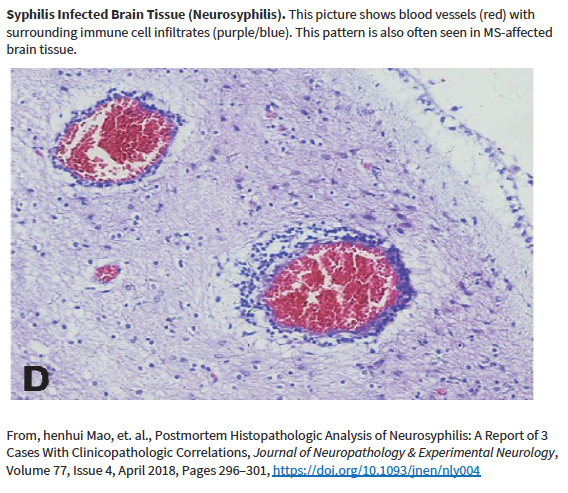
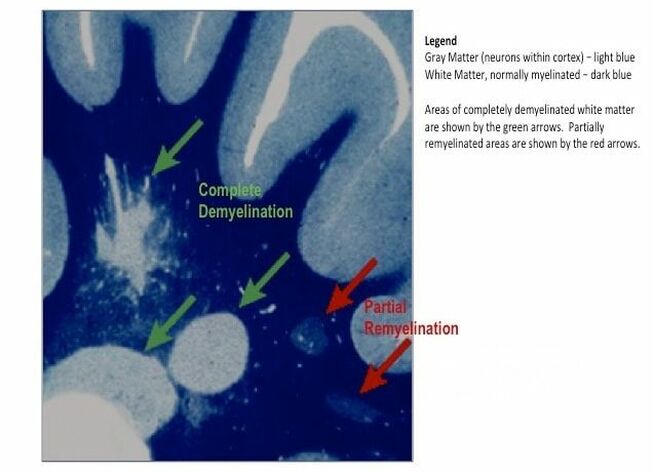
Other researchers have shown specific antibodies against bacterial peptidoglycans in the spinal fluid of MS patients. When we took our MS candidate bacteria (identified by sequencing MS brain lesions) and sonicated them, their peptidoglycans were exposed. Therefore, we likely detected antibodies against peptidoglycans in the spinal fluid of MS patients. That is, MS patients are making an immune response to bacteria and bacterial peptidoglycan in their spinal fluid. It may not matter if the bacteria are alive or dead, whether they arrive from the bloodstream or from infiltrating macrophages, their presence (and their peptidoglycans) are doing evil. The Microbial Hypothesis of MS It’s hard to imagine that bacterial antigens (proteins), including peptidoglycan, in the brains of patients with MS are doing anything but raising havoc – inflammation, macrophage infiltration, and the destruction of an important bystander, myelin. That’s right, our view at Cracking the MS Code is that the MS disease process does not specifically target myelin. It targets bacteria and their antigens within brain tissue, and myelin is damaged in the process. Myelin is a bystander, injured by the inflammatory process surrounding it. If the bacterial antigens are removed quickly and completely, myelin and their enveloped axons may be preserved without any loss of function. If there is some damage to myelin, remyelination may occur. But if all the bacterial antigen cannot be removed, or if new bacterial antigen is formed due to replication of bacterial cells, the inflammation goes on, myelin is ruined and removed, axons are lost, and neurologic deficits ensue. Neurosyphilis, Neuroborreliosis, and Neurologic Tuberculosis MS is not the only disease with oligoclonal bands (OCBs) appearing in the patients’ spinal fluid. Neurosyphilis, syphilis that affects the brain or spinal cord, and neuroborreliosis, neurologic Lyme disease, are bacterial diseases that bear some resemblance to MS. Both of these bacterial infections can be insidious and chronic. Both often cause OCBs to form in the spinal fluid. However, the OCBs are directed against the infecting bacteria, not myelin. (Remember, these OCBs are really IgG antibodies made in the brain and/or spinal cord, so, of course, they are directed against the invading microbes.) And both neurosyphilis and neuroborreliosis (neurologic Lyme disease) can cause severe neurologic dysfunction and death. These bacterial brain infections can relapse even after treatment. And in neither disease can the causative bacteria be cultured directly from the spinal fluid. Diagnosis requires special serologic or molecular testing of the blood and spinal fluid. Neurologic Lyme disease is inflammatory and can cause demyelination. The pathology of affected brain and spinal cord resembles MS in several ways, including the presence of demyelinated areas. The demyelinated area is circled (DOI:https://doi.org/10.1016/j.ajpath.2015.01.024) Neurologic tuberculosis is also interesting. This disease is “paucibacillary” (few bacteria). That is, a very few tubercle bacilli cause a terrible disease that is difficult to diagnose and hard to treat. Diagnosis requires culturing large volumes of spinal fluid for weeks or months to find the causative tubercle bacilli. If you catch them, you are lucky. Treatment is often started empirically (by a guess), and stopped later if all tests are negative. And treatment of neurologic TB requires suppression of the immune system to lessen the damage. These cases are treated with a combination of anti-tuberculosis drugs and steroids, usually prednisone. Months of treatment with these antibiotics and a slow taper of the steroids is required to control and cure the disease.
I offer this for your consideration because I want to see the bigger picture with MS: What if MS is also a “paucibacillary” disease, usually caused by bacteria, a few or many different ones, that cannot be easily cultured from the spinal fluid, that may lay dormant and recur, and that must be diagnosed indirectly by serology? Our lab at Cracking the MS Code aims to show that spinal fluid serology tests are the most effective way to diagnose and direct the treatment for developing MS. If this proves to be true, doctors could make early diagnoses and apply appropriate treatments including antibiotics to suppress or eradicate the inciting bacteria, with specific immunosuppressants to minimize the damage. Can we actually cure cases of MS? I think this is possible, but we need to know exactly what we are facing. Guessing is not acceptable. Next article: Who should we treat, and how can we do it? What are the chances of making these patients better or limiting the neurologic damage?
0 Comments
Our latest paper, published in the Journal of Molecular Medicine, shows that antibodies directed against bacteria do occur in the spinal fluid of MS patients. The paper details a cerebrospinal fluid serological study that supports the idea that some of the MS candidate microbes we’ve identified contribute to demyelination in some patients.
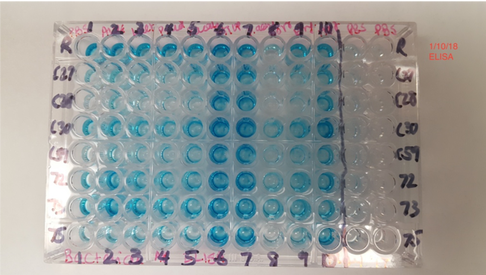
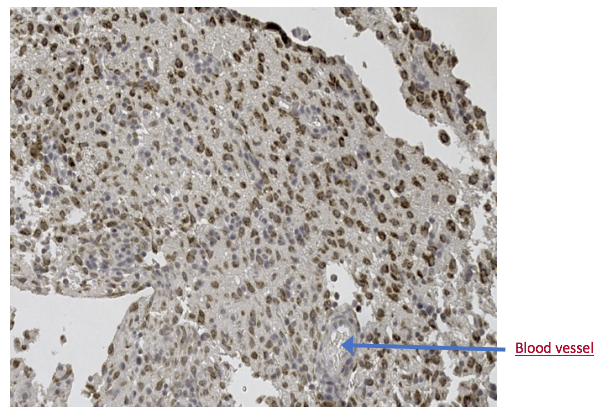
A number of infections of the central nervous system can lead to demyelination: distemper in dogs, and measles in human are just two examples. The underlying risk factors and mechanisms for microbe-driven demyelination are poorly understood, since they occur unpredictably and at variable rates. At Cracking the MS Code, we work to uncover the ways in which our “MS suspects”—Akkermansia, Atopobium, Bacteroides, Lactobacillus and others—may trigger antibodies directed against bacteria in the brain and/or spinal fluid. We appreciate your interest in our research. The work for this study was done at the University of Utah with help from ARUP Laboratories, Intermountain Health Care, and Dutch researcher Jon Laman. Future research will help establish if this new test—the spinal fluid antibody test—can help improve the treatment of MS patients. That is our ultimate goal. Link to paper: https://link.springer.com/article/10.1007/s00109-021-02085-z#citeas Interesting patient stories have emerged, as we collect clinical data for our MS study. The stories presented here are true, but are abridged or altered somewhat to avoid disclosing personal health information (PHI). PHI is protected by law, which we take seriously. But some patients have given us specific permission to talk about their cases. As for the data, we have tested ten bacterial antigens in the lab, derived from bacteria we grew in culture here at the University of Utah and at the Intermountain Medical Center Microbiology Laboratory. These antigens are our markers for MS detection. (A preprint describing this work in detail is available here.) Please see the list of definitions at the end of the article, for help with medical acronyms. The following five stories open doors into our understanding of MS. MS-10 is a middle-aged woman with progressive MS. She has experienced a multitude of neurologic symptoms starting with hearing difficulties, then difficulty walking, generalized weakness, followed by speech difficulties. She is now confined to a wheelchair. The neurologists have called her syndrome “atypical MS” with severe neurodegeneration. Spinal fluid analysis shows relatively low antibody levels and intact BBB, but a markedly elevated IgG index and several OCBs are present. This indicates that antibodies against something are being made in the brain and spinal fluid. Cracking the MS Code study result: Her spinal fluid has detectable antibodies against all ten MS bacteria, and is strongly reactive against three of the MS bacteria. However, none of these spinal fluid reactivities were higher than expected based on the reactivity of her blood serum. Comment: Although this patient’s spinal fluid has a relatively low amount of total antibodies (IgG), they seem to be directed against our MS antigens, and three in particular: Akkermansia, Atopobium, and Cutibacterium. It will be interesting to see if any of the OCBs in her spinal fluid bind to any of these MS candidate bacteria. That is, are any of her OCBs (which are, by definition, IgG antibodies in spinal fluid) directed against our MS bacteria? If so, we could reasonably conclude that one or more of these bacteria set off her demyelinating disease. MS-13 is an older pre-diabetic man who developed a tremor and hand weakness. Evaluation showed a lesion in the medulla, deep in the brain. Multiple tests for infection came out negative, but for some reason the patient was treated with eight weeks of intravenous antibiotics and steroids. The medulla lesion improved and so did his symptoms. He is no longer receiving neurology care. The diagnosis was felt to be ADEM, an MS-like disease that typically does not recur. (Multiple sclerosis recurs multiple times, whereas ADEM patients have just one demyelinating episode.) The patient’s spinal fluid IgG index was high (antibodies made within the brain), his albumin index was high (leaky BBB), but the OCB test was negative. Cracking the MS Code study result: Antibodies were detected in his spinal fluid against all six MS bacteria tested, and three of those were strongly reactive. Comparisons to serum reactivity were not possible because no serum was collected at the time of his illness. Question: What antibiotic did this man receive? Did it actually help limit his disease? Answer: We don’t know. Likely, the doctors prescribed an antibiotic that gets into the brain well, like ceftriaxone. We can’t say for sure if the antibiotics or the steroids limited his disease. We can only determine this by a well-designed study involving many such patients. MS-82 is a young woman who developed myelitis (spinal cord inflammation) following a two week flu-like illness with fatigue. She presented to the hospital with neurologic symptoms that included tingling in her legs and difficulty walking. She had a spinal tap just two days later. MRIs showed areas of demyelination in both the brain and spinal cord. Spinal fluid studies showed a leaky BBB, but no elevation in spinal fluid antibodies and no OCBs. She improved somewhat with aggressive immune suppression. She has a relatively severe form of demyelinating disease with MS-like symptoms involving both the brain and spinal cord. Cracking the MS Code study result: This patient has detectable antibodies against nine of the ten MS bacteria, two strongly reactive (Lactobacillus, Cutibacterium). We also see evidence for extra antibodies in the spinal fluid (intrathecal antibody production), compared to those found in her blood, against five of these bacteria. Question: Are the antibodies in her spinal fluid, particularly those made in excess, directed against anything in particular? Answer: Yes, they seem to be directed at some of the MS antigens we tested. We applaud the doctors for stepping in so early to help this patient. It seems likely that she may have now developed an immune response in her spinal fluid (i.e. OCBs), and that the first test was done too soon—after only two days of neurologic symptoms—to detect the bands. It would be ideal to retest her spinal fluid and see if the antibody response or OCBs have developed. MS-03 is a relatively young Idaho rancher whose case proved to be uniquely interesting. This man and his family have been outspoken about his disease, both in the local media and as the subject of an ESPN feature video. The inciting event was believed to be an attempted winter rescue of a distressed newborn calf, possibly leading to inhalation of aerosolized bacteria in the enclosed pickup truck. This was followed by a brief febrile illness and then progressive neurologic dysfunction over the course of several weeks, with ADEM as the final neurologic diagnosis. Sequencing of a small diagnostic brain biopsy of this patient revealed lactobacillus as the most abundantly mapped microbe at the genus level. Lactobacillus is found in the female genital tract of many mammals, including humans, where it is dominant, and in cattle including newborn calves. Cracking the MS Code study result: This subject’s CSF was highly reactive against five of the tested bacterial antigens, with the highest EI against Lactobacillus paracasei among all the subjects tested. However, the spinal fluid antibodies against lactobacillus were not higher than expected, arguing against a causative role for lactobacillus in his disease. In an attempt to prove causation, OCBs were retested, but they did not survive prolonged freezing. Comment: It is plausible that this subject’s ADEM was triggered by microbes, including Lactobacillus, encountered from an exposure to a newborn calf. However, the data is not completely conclusive. The patient has recovered as much as possible, but still has some difficulties with activities. He continues to coach basketball and live with his family. The blue color indicates binding of antibodies in spinal fluid to the antigens on the plate (i.e. CSF reactivity). The preliminary testing shown here was not used for the final analysis. MS-85 is a young woman who presented to the hospital with a new onset of seizures. Her seizures were unprovoked and not associated with fever or medication use. They were generalized (“grand mal”) and witnessed by her family and then the hospital staff. Brain MRI imaging showed a “temporal lobe T2/FLAIR hyperintensity” and “additional predominantly frontal white matter T2/FLAIR hyperintensities.” Spinal fluid testing showed an elevated IgG Index and numerous OCBs. BBB was intact and total IgG in the spinal fluid was normal. Apart from seizures, this woman had no neurologic signs or symptoms.
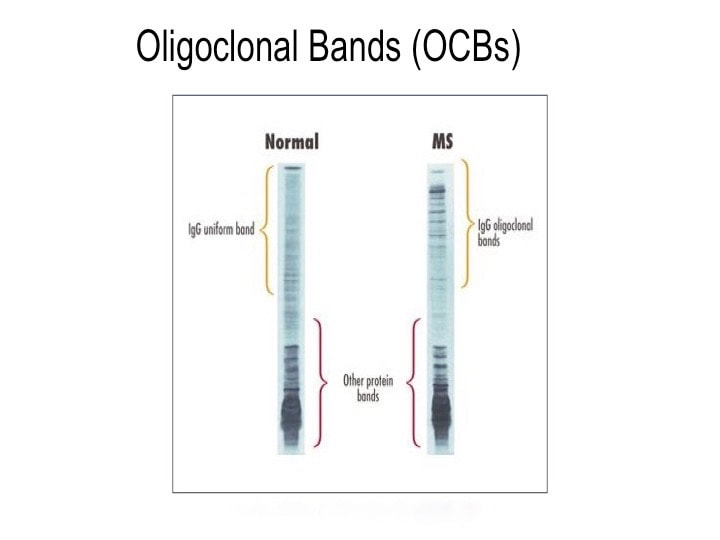
Cracking the MS Code study result: This patient’s CSF was reactive against all ten tested bacterial antigens, and strongly reactive against Cutibacterium and Akkermansia. Spinal fluid reactivities were higher than expected based on the reactivity of her blood serum against six of the MS candidate bacteria. CSF reactivities were 50-60% higher than expected against Lactobacillus, Pseudomonas, and Bacteroides. Comment: About 10% of MS cases present with seizures. A focal lesion in the temporal lobe is the likely cause of MS-85’s seizures. The areas of frontal lobe demyelination likely went unnoticed, or the symptoms were subtle (e.g. cognitive difficulties) and were well compensated. Testing suggests that the IgG immune response in her CSF is directed against several MS candidate bacteria. This is evidence that bacteria (particularly Lactobacillus, Pseudomonas, and Bacteroides) played a role in her demyelination and seizures. Despite all this, she has done well without specific treatment of her MS. Conclusions It’s clear from the above cases that demyelinating diseases present in a variety of ways, with different symptoms and outcomes. It is also clear that the spinal fluids of patients with demyelinating diseases have antibodies directed against some or most of our MS candidate bacteria. In some cases, the levels of these antibodies are higher than expected. Sometimes we can show that these anti-bacterial antibodies were made in the brain and spinal cord, and did not just leak in from the serum, especially in cases where the BBB is intact. These findings may be illuminating the causes of MS. The facts don’t lie, and it’s up to us to interpret them correctly. Acronyms and definitions Demyelination = the process of myelin destruction in the brain and spinal cord. Myelin is a fatty substance that acts as insulation between nerve fibers. BBB = blood-brain barrier. This normally functions to keep cells, toxins, and big molecules like antibodies in the blood and out of the brain and spinal fluid. IgG = Immunoglobulin G. These are the main forms of antibodies found in human spinal fluid. MRI = magnetic resonance imaging. This is the brain imaging technique generally used to show demyelination and confirm the diagnosis of MS. NMO = neuromyelitis optica. This is an MS-like disease that tends to be severe, usually involving the spinal cord and optic nerves. ADEM = acute disseminated encephalomyelitis. An acute MS-like illness that occurs only once and does not recur. OCB = oligoclonal bands. These are IgG antibodies found in the spinal fluid and not in the blood. They consist of a few (oligo) clones (species) of antibodies visualized on a gel (bands). They are direct evidence of antibody production in the brain or spinal cord. Their intended targets remain unknown. Some researchers believe they are the key to finding the cause of MS. IgG Index = a calculated value that compares IgG antibodies in the spinal fluid and serum to albumin in the spinal fluid and serum. Elevated values of the IgG index are evidence of antibody production in the brain or spinal cord.
Propionibacterium (now Cutibacterium) acnes, Lactobacillus, Borrelia burgdorferei, and a few others. (This work is covered by the provisional US Patent Application #62785377, filed 12/27/18.) We took cerebrospinal fluid (CSF) from 14 patients with MS (and other demyelinating diseases) and compared the CSF to a set of four controls who had no active infection at the time of collection. Among these 14 patients, the CSF from seven reacted against Akkermansia. This includes one patient whose first CSF sample did not react but a second sample, taken three weeks later, was strongly reactive. This patient developed anti-Akkermansia antibodies in his spinal fluid over the course of a few weeks, one chilling aspect of the process of this terrible disease. Interestingly, we also collected spinal fluid from three patients who had had brain biopsies, so we had some idea about what microbes we might find. Two of these three patients had RNA sequences that mapped to Akkermansia. We detected anti-Akkermansia antibodies in their CSF, more than five years after their initial attack and brain biopsy. (Antibodies are stable and long-lasting but they are cleared in months, not years.) For the other patient, we did not detect Akkermansia in the brain tissue, and no antibodies against that bacterium appeared in the cerebrospinal fluid. My team believes our evidence shows Akkermansia may be the cause of ongoing MS in research subject 019. That's one patient, with one traceable link to a bacterial cause of MS. Yes, the numbers are small—the number of MS patients whose CSF we examined, the number of controls, and the number of studies completed—but the findings are compelling so far, and worth exploring. My colleagues and I want to examine the oligoclonal bands (OCBs) in those two patients with Akkermansia RNA and antibodies in CSF against it. With time and funding, we hope to do exactly that kind of encompassing research. You won’t be surprised to hear that testing for oligoclonal bands requires specialized techniques involving the absorption of antibodies in cerebrospinal fluid. Very expensive tests that are, again, so worth pursuing. Could the oligoclonal bands prevalent in MS patients be directed against bacteria? Sure, no one has tested for it. We plan to. The field begs for extensive inquiry and research. Still, even if the OCBs are proven to be directed against bacteria, we can’t guarantee that treating those bacteria directly will improve the disease. We might, as with neurosyphilis or neurologic Lyme disease, be able to limit the damage. But often the neurologic deficits do not reverse. In full disclosure—and if you’ve read this far, you are able to withstand the bumps and bruises that medical research delivers—bacterial infections in the brain may actually “trigger” MS, setting off the disease, then vanish like a ghost. Or those bacterial infections might stay and fester, causing progressive MS. Or new infections might occur, causing relapses of existing MS. In every one of these instances, case-specific bacterial diagnostic tools would be very helpful in our campaign to help MS patients. I will be pursuing this bacteriological line of inquiry in my research and in this blog until the real culprits of MS step forward to claim their ignominious prize: the causal actors in MS. You can help by donating to our MS research, writing to the National MS Society about the importance of this research, and sharing this blog on social media. The more minds we get searching for the cause of MS, the better. Thank you for your interest! All images are from our Microbes in MS research lab.
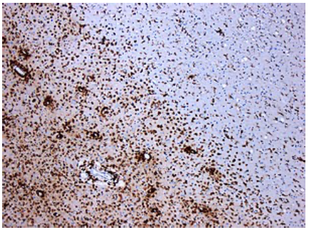
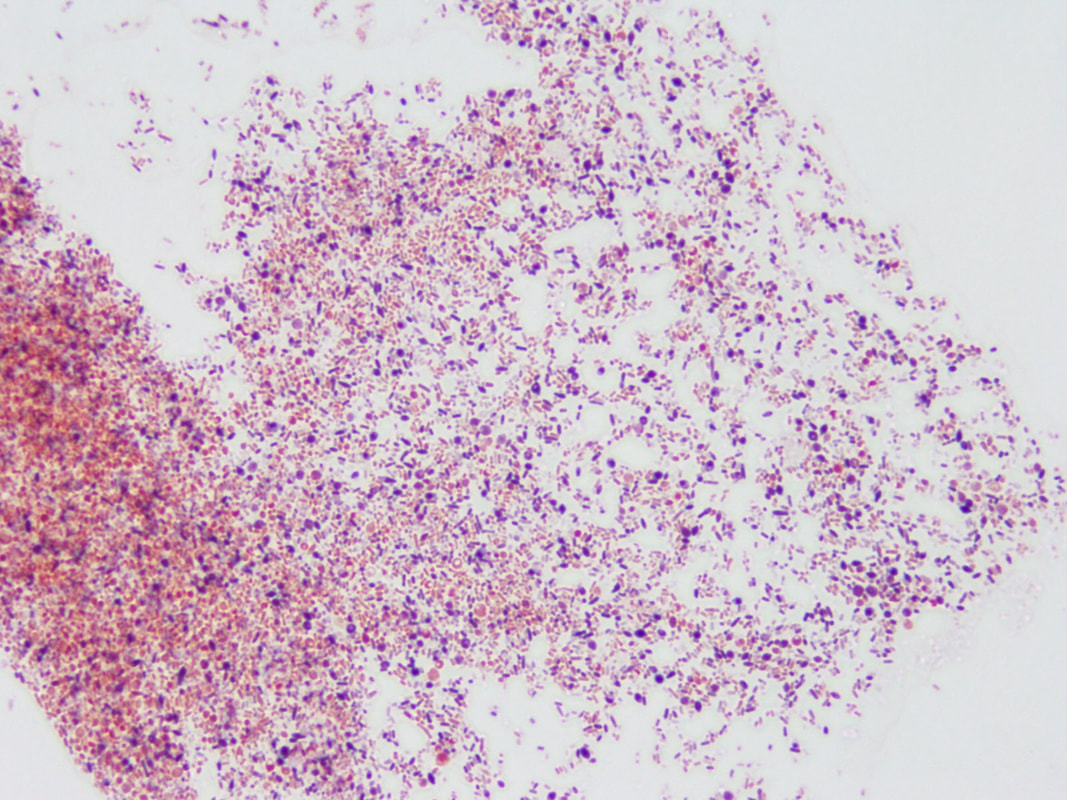
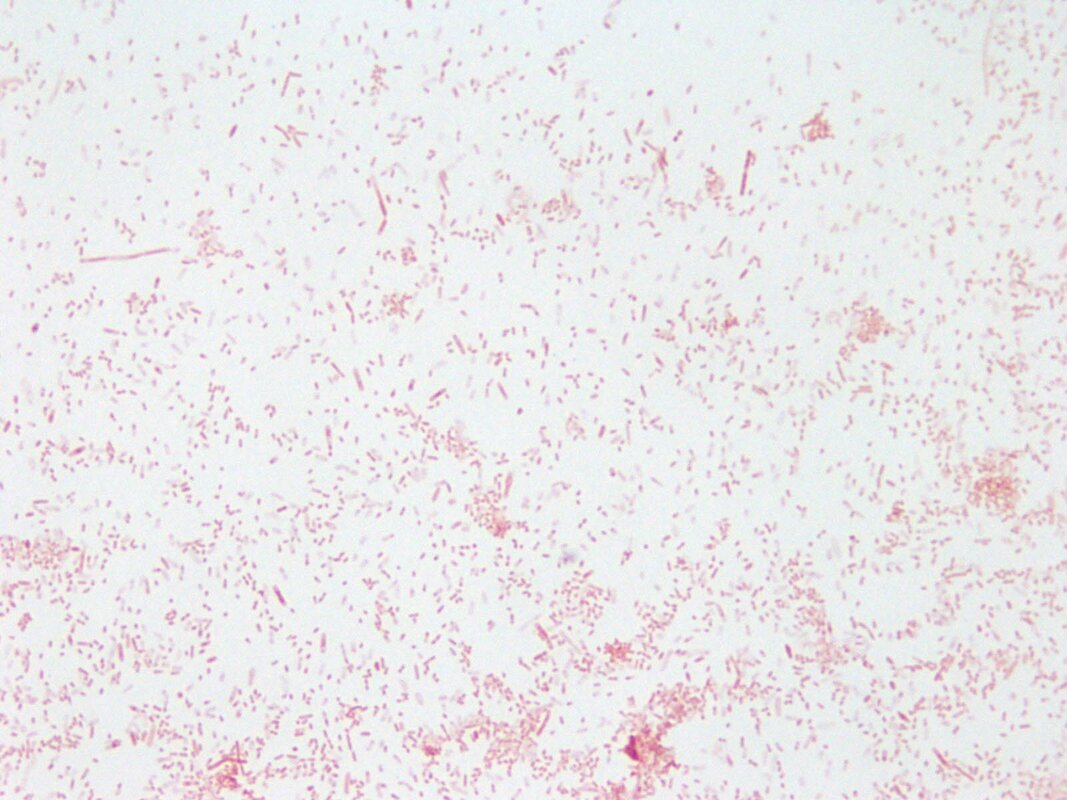
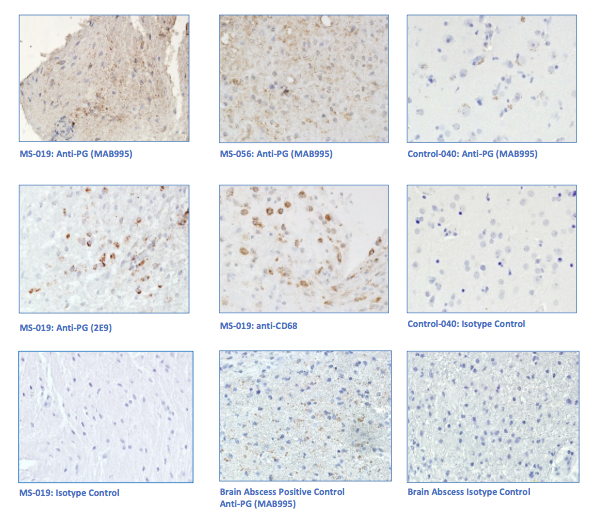
“primary demyelination” brain specimens from MS patients are full of macrophages, and that these macrophages appear to be “activated.” Macrophages—which start as monocytes in the bone marrow, move into the blood, and are recruited into areas of infection or damage--are a mop up team. And luckily for us in MS research, macrophages also work as antigen presenters. That is, they are drawn to areas of infection, they engulf and digest the offending microbes, and they “present” pieces of these microbes to the immune system. While macrophages can also be found in areas of damaged tissue, say after a crush injury or a burn, our interest lay in the macrophages in MS brain lesions. Figure: Macrophages stained within an MS lesion. Most experts in the MS field believe macrophages are bad news. They say that macrophages are drawn into MS brain lesions by the autoimmune process where they serve to damage tissue, harming people and doing no good at all. Because bacteria are not present, traditionalists hold, the macrophages must be a guerrilla SWAT team bent on brain destruction. (If this were true, scientists should be able to limit tissue damage and demyelination in patients by inhibiting macrophages, resulting in improvements in MS. However, drugs acting directly against macrophages have never been developed and tested, so we don’t know what their effects might be.) But my team and I wonder: What if macrophages are just doing what they are supposed to do—getting into infected areas to clean up and stimulate the immune system for a lasting fix? What if they are not the problem but, rather, a part of the solution? A special technique called immunohistochemistry has helped us see the bacteria—or at least parts of them—in MS brain lesions. Here’s how the science works. Peptidoglycan (PG) is an important component of bacterial cell walls. PG isn’t found in human cells. In fact, the human body raises up a special antibody against bacterial peptidoglycan. This “anti-PG” antibody binds to areas where peptidoglycan is present, so we can use it to detect the presence of PG in tissue, blood, or spinal fluid. In a convoluted series of steps, involving anti-PG antibodies and a few chemical cohorts, we can visualize PG on a slide. As with any good lab work, a set of controls proves that the reaction we see really is detecting bacterial peptidoglycan in human tissue. We tested as many MS brain samples as we could for PG using this method, called immunohistochemistry. We did not have too many samples to use because MS brain biopsy samples from living patients tend to be few. All four MS samples tested were positive for PG, with a much different pattern of PG reactivity seen in the epilepsy controls. Our group was not the first to apply immunohistochemistry to detect PG in MS brain lesions. Jon Laman from the Netherlands and Chris Power from Canada have also done it. In fact, we borrowed one anti-PG antibody from them, and bought another from a commercial supplier. Small groups of dedicated researchers are exploring the MS question as thoroughly and deeply as we can. We work together. We seek funding as well as scientific support for ground-breaking MS insights. If you’d like to take a look at bacterial peptidoglycan within MS brain plaques, you can get your science nerd on below. Figures: Immunohistochemical Analysis of MS Brain Tissue. These six photomicrographs show formalin fixed, paraffinized brain tissue sections from MS subjects, epilepsy controls, and a deidentified brain abscess control. The reddish or brown color shows detection of bacterial peptidoglycan (PG). Brain tissue from subject MS-019 shows specific staining with two different anti-PG antibodies. PG signal is also seen in MS-056 and faintly in epilepsy control subject 040. (The images are adapted from our February 4, 2019 article in Scientific Reports.)
So, in conclusion, and within the constraints of time, research funds, and available tissue samples, macrophages are in MS brain lesions and so is one important component of bacteria – peptidoglycan. The team showed previously that most MS brain biopsy specimens contain bacterial sequence different from the controls (read the sequencing blog here). So the scenes of the crime in MS patients’ brains contain bacterial RNA, bacterial cell walls (PG), and macrophages (either responding appropriately or causing trouble). Can we ever tell what really matters? Yes, we can, by measuring immune responses within the spinal fluid and blood. If those bacteria in MS brain samples are the root of trouble, macrophages will present them to the immune system. B lymphocytes and specific antibodies against those bacteria will be made in the brain and will collect in the spinal fluid. Which is the topic of our next blog: how the team grows bacterial candidates in lab culture, and what results that lab work has already delivered.
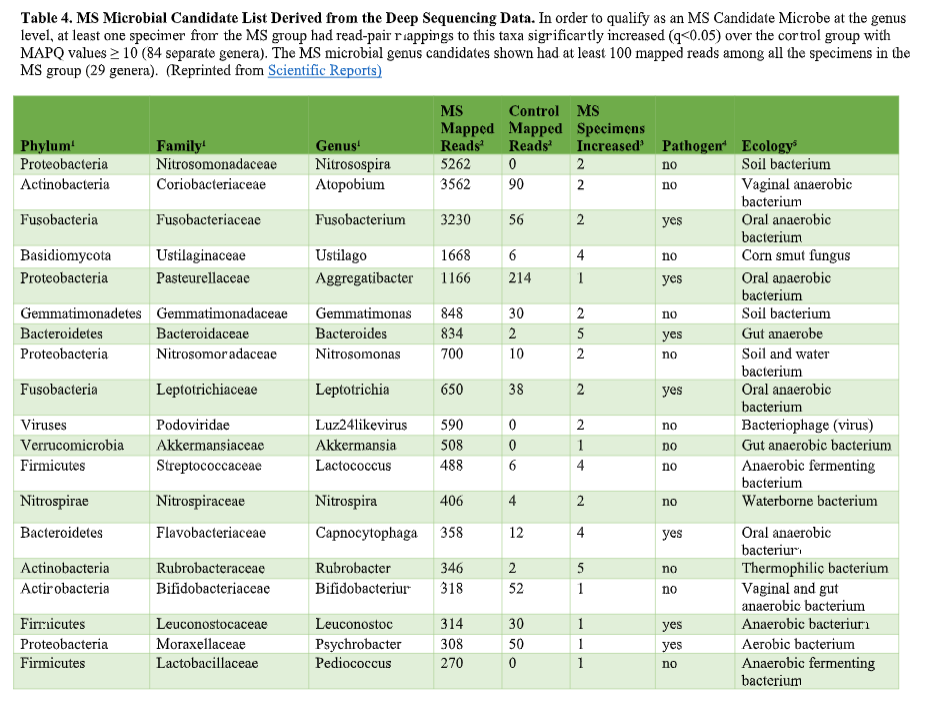
That’s where the Microbes in MS research team is diving into deep, uncharted waters. We’ve seen that RNA sequences within MS brain lesions differ significantly from their epilepsy control counterparts as reported in Scientific Reports. Twenty-nine MS candidate microbes were identified. We know that the MS lesions we studied contain more bacterial sequences; they’re “dirtier” than the controls. Further, most of these MS candidate microbes happen to be anaerobic bacteria. That is, MS seems to be related to (fussy!) bacteria that live primarily in areas lacking oxygen. Emily Eckman is busy working in our laboratory to grow and validate some of these microbes so that we can develop tests against them. Anaerobic bacteria, found commonly in the gut, mouth, skin and vagina, generally go about their business as a normal part of the microbial world. But they sometimes cause infections. These anaerobic infections can be both indolent (progressing slowly) and polymicrobial (having multiple infectious agents), which makes detecting them complex. That’s the power and beauty of deep sequencing: It can reveal multiple bacteria in a lesion and show us the anaerobic bacteria that don’t grow well in lab cultures—the so-called “unculturables” or “uncultivatables.” Some of these microbes are found only in mixtures with their friends, who supply some of the necessary nutrients. Here is the MS Microbial Candidate List derived from the Microbes in MS team’s deep sequencing data: Which of these MS microbial candidates do we believe might actually be contributing to the disease? It’s hard to know a priori, but some candidates are more attractive (if bacteria can actually be “attractive”) than others. For instance, #2 on the list, Atopobium, is found mainly in the vagina. If we can confirm this finding in further research, the presence of Atopobium could help explain the female predominance of MS. Since men don’t have vaginas, they could not get at least some strains of Atopobium, and they don’t have MS nearly as often. However, Atopobium has been described as a contaminant in other sequencing studies, so it may or may not be a real finding. Another “attractive” MS candidate is Akkermansia, because it was shown by another group in another study to be more abundant in the stool of MS patients compared with controls. We found this anaerobic human gut bacterium in brain samples, not stool samples, but the parallel is interesting. We did not find Akkermansia in the controls, at all. Likewise, the most abundant MS candidate microbe, Nitrosospira, also was not found in the control specimens. Neither Akkermansia nor Nitrosospira are believed to be common contaminants, and the best defense against contamination is to run controls—which we did. How can we know what microbes may be causal for MS, and which may be spurious? There is a way. Stay tuned for the next blog topic: Macrophages and Immunohistochemistry. It may not sound like riveting stuff, but I’d start popping the popcorn now. Shutterstock bacteria illustration ID: 734835721
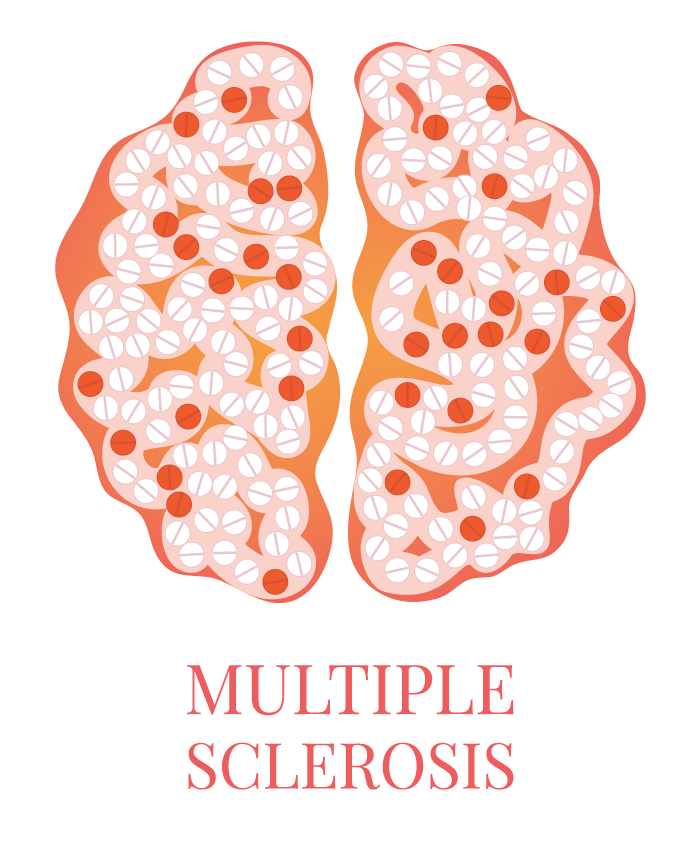
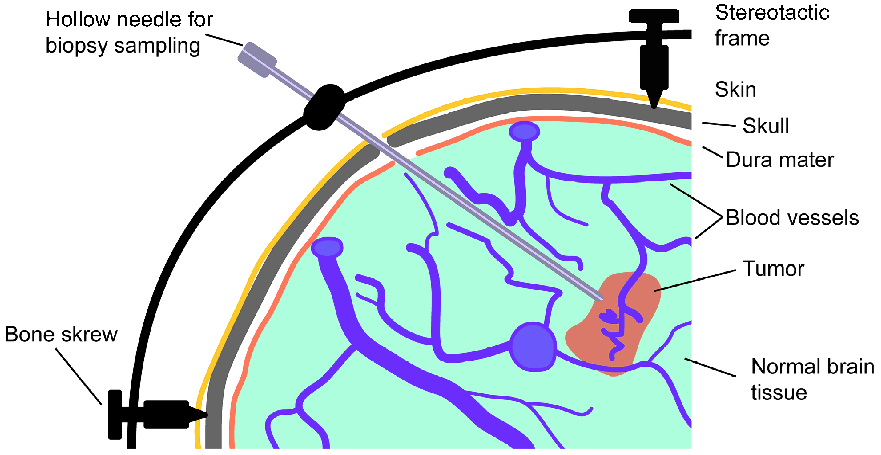
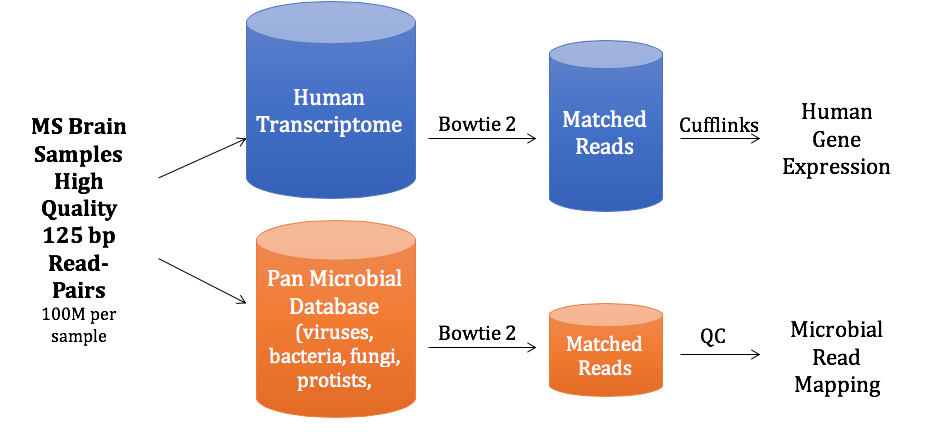
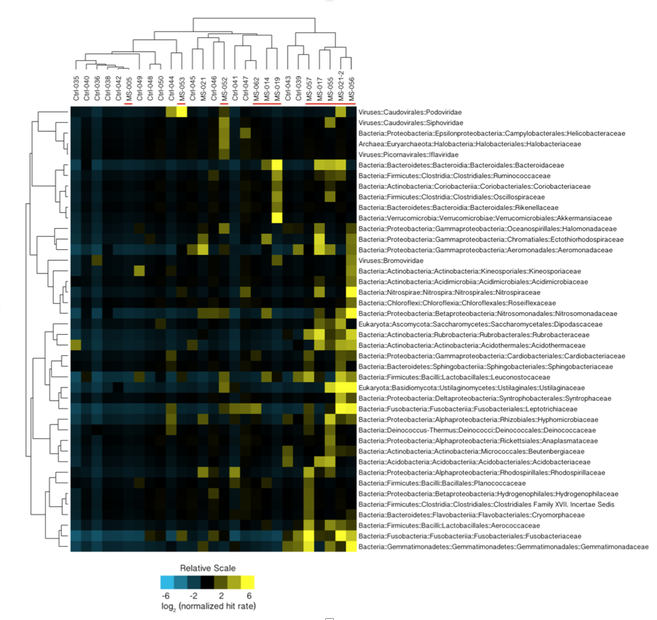
You’ve heard how soil samples can provide a deep look into the history of a landscape. Deep sequencing does the same with human tissue. This metagenomic testing provides unbiased “reads” of DNA or RNA sequences from the sample provided. It’s a complex process as you might guess, given the complexity of our human biosystems. When I tried to explain my research to my teenage son, he said it seemed like a crap shoot trying to find the cause of MS. I said, “It’s more like a wet spaghetti test: You throw your data up against the sequencing wall and see if it sticks.” Deep sequencing uses algorithms to splice together parts of RNA into whole strands called “paired-end” reads. As sequencing technology has improved, the reads have gotten longer and more precise. Here's a helpful illustration from Illumina's website: Why study RNA and not DNA? Simple. DNA is stable, RNA less so. DNA carries the genetic code so it must be stable and long lasting. RNA is made for a temporary purpose—messages, ribosomes, and other stuff—then it degrades and goes away. DNA is everywhere including the walls and ceiling of your house, on office machines, and even floating around in the air. RNA exists only within DNA and thus has less risk of contamination. So the goal of our sequencing studies is to see what microbes might be inhabiting diseased brain tissue from patients with MS. That is, if MS is related to one or more microbes—viruses, bacteria, fungi, protozoa, and/or archaeans—those microbes may very well be found at the site of the disease, the demyelinated areas within the brain. Now MS patients do not routinely have brain biopsies. MS can usually be diagnosed based on neurologic symptoms, brain MRI findings, and, sometimes, spinal fluid tests. But occasionally, doctors dispute a patient’s diagnosis. For instance, one University of Utah patient with pre-existing MS suddenly suffered a drastic downturn, such that she could no longer speak or walk. Her MRI showed a single, new, very large periventricular lesion (affecting a ventricle of her brain). Was it brain cancer or a glioblastoma (a malignant nervous system tumor)? Only a biopsy would tell. Her brain biopsy showed no cancer, just “primary demyelination”—the hallmark of MS. We know this because we sequenced her brain biopsy. Happily, she revived quickly and over the next few months, she returned to her previous quite functional state. We have no idea why. The procedure for a stereotactic brain biopsy is depicted. Each MS patient in our study had one of these procedures. Over the years, our Microbes in MS research group at the University of Utah has collected about 20 such cases where a patient’s brain was biopsied looking for cancer, and the result was “primary demyelination.” We decided to put these samples to the spaghetti test and map the reads looking for microbes. Our first deep sequencing study, in 2012, produced 36 base pair (bp) single end reads, and about 10 million reads per sample. These reads represented RNA extracted from diseased brain tissue from several patients who died with MS, and similar normal brain tissue from control patients without any brain disease. For the first time anywhere, the testing identified the virus GBV-C in one patient’s brain tissue. Unfortunately, no other brain specimens we studied contained this virus, so we do not believe that GBV-C has anything to do with MS. That’s the nature of research, try and fail and fail and try again. A word about controls. An ophthalmology professor and friend once said, “Controls are everything.” That was some good advice. Ideal controls for our study would be the brain tissues from patients with no neurologic diseases who were about the same age and sex as the MS patients. But we do not live in an ideal world. Normal patients don’t get brain biopsies. Normal tissues are available from deceased persons, in fact they are readily available, but RNA degrades quickly after death, as it should. We had to turn elsewhere. Our neuropathologist had a suggestion: Why not use epilepsy brain samples? Some epilepsy patients have seizures that only brain surgery can stop, so brain biopsies are readily available. But there is a complicating factor. The seizure surgery requires two steps: first, mapping the part of the brain causing the seizures by placing electrodes there, and second, the brain biopsy itself. This two-part surgery can allow for more microbial contamination, so more microbes (and more microbial sequences) are expected in the epilepsy control specimens compared to the MS group who had only one surgery—their biopsy. Even so, using epilepsy brain biopsies as controls turned out to be a great idea . . . or at least the best idea we could come up with. Monks in the machine. The output of this sequencing process is paired-end sequences. Lots of them. So many, in fact, that it would take a castle full of monks thousands of years to do all the math. Fortunately, much to the relief of the monks, we have supercomputers to do the comparisons of about 150 million reads per sample, more than a billion overall, per patient. Sequencing Output and Analysis Scheme We compared 12 “primary demyelination” (i.e. MS) samples with 15 epilepsy controls. One of the first things we noticed was that the MS samples were “dirtier” than the controls. That is, they had more microbial sequence mappings (128 per million sequences) than the controls did (77 per million). Much to our surprise. And scientific delight. What were all those microbes up to? We then used a technique called the False Discovery Rate (FDR) to see what microbes might be enriched in the MS brain specimens. This required some normalization of the sequencing data so we could compare specimens directly. Since we don’t believe that MS is caused by any single microbe, we had each MS specimen compared to the set of controls. The “cluster” figure from our findings on the “heat map,” below, shows overrepresented (significantly higher expression) microbial families in each row. Each column represents a different sample: either MS (red bars) or epilepsy controls. The computer application (Cluster 3.0) arranges the microbial families and the samples by their mathematical relatedness, allowing us to see what goes together. Yellow is hot (overexpression), blue is cold (under-expression), and black is in the middle (median). Notice the cluster of 4-5 MS samples on the right side of the figure. All those yellow boxes show that these specimens were particularly “hot” with an increased number of sequences derived from multiple microbes. Our Microbes in MS "HEAT MAP" Results—Ten of the 12 MS brain samples we studied had at least some microbial sequence enrichment at the genus level. Once we stopped smiling, and jumping around the lab, we had the machine monks do some additional filtering of the data, leading to a list of MS candidate microbes. Additional filtering led to a manageable candidate list with 29 microbial members. Not all of these MS candidate microbes are likely to be real contenders but some are worth discussing. For instance, Fusobacterium, Aggregatibacter, and Bacteroides are well known human pathogens. All three can get into the bloodstream; this is called bacteremia. Another contender, Atopobium, raised our eyebrows and our pulses. It’s part of the normal vaginal anaerobic flora and is not a known pathogen—but having vaginal bacteria appear in the brain—we know not how—could explain a lot since two-thirds to three-fourths of MS patients are female. (Neuroscientists recently found gut bacteria inhabiting the cells of healthy brains, a preliminary study which one UCLA doctor called “mind-blowing.”) Do we believe all of these MS microbial candidates are real culprits in causing MS? No. It’s hard to imagine how Ustilago, the corn smut fungus, could get into people and cause disease. Likewise, Nitrosospira and two related genera are common environmental organisms. We used the controls and fancy statistical comparisons to filter out spurious findings but there were millions of sequences compared to thousands of microbial taxa. And so the research team, and the monks, have to keep an open mind. If some spurious microbes found their way into the data, we will sleuth them out. Stay tuned for the list of our winning microbial candidates in my next blog, and for a future blog on antibacterial immunohistochemistry—another way to gain new and supportive research evidence to help find the cause of MS. Shutterstock ID: 777790381
It includes proteins, electrolytes, antibodies, hormones, and sometimes outside agents like drugs and microorganisms. Doctors sometimes examine the antibodies present in patients’ serum to search for invading germs. And serology is a lousy way to diagnose disease. It’s inexact and not always timely. Antibodies are those little Y-shaped proteins made by the immune system (B-lymphocytes to be exact) that stick like glue to foreign invaders like viruses, bacteria, and other microbes. But they keep their cards close to their chests. For instance, Lyme disease takes weeks or even months to make antibodies against Borrelia burgdorferei, the bacterium that causes it. And infections with other bacteria can cause false positive tests for Lyme disease—even if those other infections occurred in the distant past. Call it “diagnostic archaeology,” because who really cares if you had Rocky Mountain spotted fever or some other infection years ago? You either died from it, or resolved it and live on. In multiple herpes cases, I have helped patients who were infected with herpes simplex virus (because I cultured it), yet their blood never made antibodies against the virus. Are those patients still infected with herpes or not? Actually, it’s not clear. You can see how how serologic diagnosis can lead to problems: over-diagnosis (false positives, as in Lyme testing) and under-diagnosis (false negatives, as in herpes testing). Sometimes serology tests are the best we can do. The syphilis agent, Treponema pallidum, has never been cultured in the lab. So if you want to figure out whether a patient has syphilis or not, you have to rely on serologies. And sometimes you just have to guess because patients with primary (the earliest form of) syphilis often don’t have a positive serologic because their immune system hasn’t had time to develop antibodies against the syphilis bacterium. Antibodies come in several forms. The most important is immunoglobulin G (IgG), which accounts for most antibodies in the blood and tissues. Other antibody types include IgA, which present in the gut and other mucosal surfaces, and IgE, responsible for many allergic responses. IgG immune responses to an infection are usually polyclonal, derived from many B-lymphocyte clones, each making slightly different antibodies, but all directed at one target. In contrast, B-lymphocyte cancers (myelomas) make only a single antibody species—a monoclonal. Oligoclonal (OCB) responses are neither monoclonal or polyclonal, but something in between with a few (oligo) B-lymphocytes producing a limited number of antibodies. OCBs are everywhere in MS patients’ spinal fluid and we have no idea why. Yet. Spinal fluid in healthy people contains very few antibodies—typically about 1/1000th the level in the blood. Past MS researchers tried to detect antibodies to a number of microbes, both in blood and spinal fluid. Wally Tourtellotte at UCLA led this effort, looking for antibodies in the spinal fluid of MS patients and comparing them to those found in the patients’ blood. To make a very long and complicated story short, his team basically found that MS patients are a little better at making antibodies compared to healthy control patients. But MS patients are not enough better at making antibodies to really account for their disease. And high levels of IgG antibody levels are often found in the spinal fluid of MS patients.
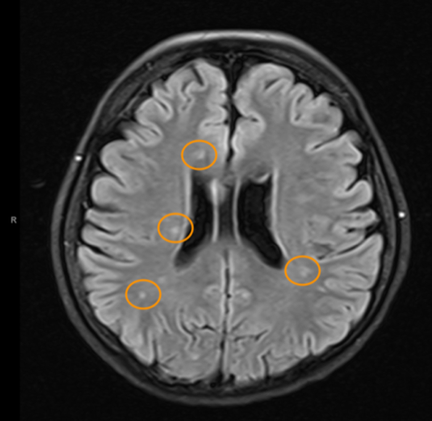
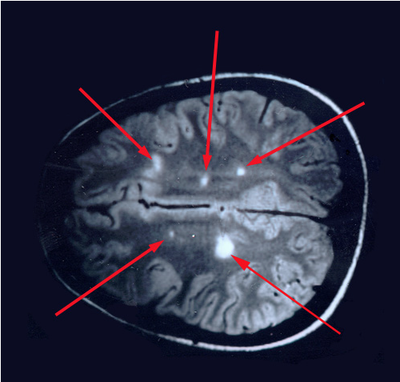
So what does spinal fluid serology have to do with MS? Everything, actually. Because “oligoclonal bands” are a terrible, confusing, and wordy way to describe IgG antibodies that collect in the spinal fluid. That’s right, most MS patients make IgG antibodies in their brain or spinal cord, and these collect in the spinal fluid. We can detect the presence of oligoclonal bands by a specialized test that uses an isoelectric focusing gel. Since all IgGs are about the same size, we use an electric charge to separate and visualize different antibody clones (also called species). These oligoclonal bands in the spinal fluid of MS patient are a hallmark of the disease – 90% of MS patients have them. When I was a medical student at Washington University in St. Louis in the 1980s, neurologist Charlie Trotter made big waves. He helped show that OCBs are commonly found in the spinal fluid of MS patients. At first, everyone believed these OCBs would be directed against myelin or some other human protein found in the brain, confirming the autoimmune nature of MS. Wrong! Sorry folks, these OCBs are not directed against myelin, myelin oligodendrocyte glycoprotein (MOG), or any other neural protein. In fact, 30+ years later no one really knows what these ever-present oligoclonal bands are doing. Oh sure, you can turn up the gain and show that some bands (antibody species) bind to Epstein Barr virus, or human herpes virus 6, but don’t believe that that’s the whole OCB enchilada. The actual targets of OCBs are still largely unknown. My lab team at the University of Utah intends to find out. Many MS researchers now believe that these OCBs are “poorly directed.” That is, they have no specific target but are produced haphazardly by the autoimmune process. I say these researchers are jumping to hasty conclusions. Because OCBs can also be found in the spinal fluid of patients with other diseases, particularly those involving neurologic infections. That is, patients with trypanosomiasis (African sleeping sickness), herpes simplex encephalitis, HIV, neurologic Lyme disease, neurologic syphilis, and neuroinvasive West Nile virus infection all have OCBs in their spinal fluid. It is logical that the targets of these OCBs are infecting or triggering organisms. It is predicted that the oligoclonal bands in specific MS patients are directed against microbes found in the brain, which our research has shown are often anaerobic bacteria (see the Microbes in MS sequencing paper recently published in Scientific Reports). MS patients need doctors and researchers to answer the biggest question: What are these tangled OCBs attacking in MS patients? Why are they there? What is their calling? Because once we know what the oligoclonal bands are aiming at, we can begin to intercede effectively. Said another way, the targets of these oligoclonal bands are the key to discovering the cause of MS. Royalty-free stock illustration ID: 81342454 The journal Scientific Reports published the Microbes in MS team’s article titled “Spectrum of Microbial Sequences and a Bacterial Cell Wall Antigen in Primary Demyelination Brain Specimens Obtained from Living Patients” on February 4th. This Q & A reveals what the groundbreaking research paper contains. Q. What did you find? A. We discovered microbial (mostly bacterial) RNA sequences that are enriched in “plaques” (sites of diseased brain) taken from patients with multiple sclerosis (MS) compared to controls. One of the main findings from the study is that the MS samples were “dirtier” than the control samples. That is, on average, there were more RNA sequences mapping to microbes in the MS samples than in the controls. MS PlaquesAnother main finding of the study is that no single microbe is associated with the MS samples. That is, MS is not as simple as other neurologic infections (e.g. syphilis or herpes), but is much more complex. Most of our MS study subjects (10 of 11) had enriched sequences from several microbial taxa. There was some overlap, and the new paper specifically describes 29 MS Microbial Candidates. Surprisingly, many of these candidates are anaerobic, along with other kinds of bacteria. We suspect that these bacteria are playing a role in the disease process we call MS. Q. Why do RNA sequencing? What is so special about that? A. Sequencing allows us to see most or all RNA species in any given sample. That is, we can identify microbial sequence from microbes that are either 1) not viable due to the tissue processing or 2) unculturable altogether. Many such “unculturable” (or uncultivable) microbes exist; perhaps a majority of the microbial world has never been specifically isolated in culture. Another advantage of RNA sequencing, besides the microbial analysis, is that it allows us to look at human gene expression, too. Q. Do MS patients usually get brain biopsies? A. No, most MS patients never need a brain biopsy. However, occasionally there is some confusion about the diagnosis. Most of our subjects had a brain biopsy because their doctors suspected a malignancy (cancer). We were fortunate to find patients like these who had “primary demyelination” as their pathologic diagnosis. Most of these patients turned out to have MS in one form or another, although sometimes their doctors disagreed about the actual diagnosis. mri from a patient with msQ. Why did you see a need to run control specimens? Where did you get the controls? A. We felt that controls were very important because the MS brain specimens we studied are not sterile. That is, when these patients had their brain biopsies, the tissue was preserved in formalin, then embedded in paraffin wax in preparation for viewing under the microscope. While this process does preserve the architecture of the tissue and, fortunately, its RNA, it is not sterile. The control specimens were taken from patients with epilepsy who needed to have a piece of diseased brain removed to treat their seizures. The control brain tissue specimens were handled in the same way as the MS brain specimens. This allowed us to compare the microbial sequence content of each MS brain specimen with a set of 15 controls. This was done with each sample at more than 10,000 microbial taxa. Special statistical testing helped us draw some conclusions. Q. How do you know that these “MS Microbial Candidates” are meaningful? Couldn’t they just be an artifact of the preservation or sequencing process? A. We are not yet completely sure how meaningful these findings will be to the MS field. However, other groups have also shown a relationship between microbial components in the brain and the presence of MS. Couple this with the emerging view that microbes might play a role in normal functioning of the brain, and the team realized what a rich field of research this could be. We confirmed the presence of “peptidoglycan” – a component of bacterial cell walls – in several of our MS brain specimens. This leads us to believe that not only is microbial sequence (RNA) enriched in MS brain lesions, many of these lesions also contain bacterial protein (peptidoglycan). This could plausibly complete the link between microbes in MS brain tissue and the disease process. Q. What do you think about MS as an autoimmune disease? Is this view of the disease actually correct, or should we think about MS in some other way? A. The conventional view of MS is that it is “autoimmune,” where the human body is attacking itself, leading to “plaques,” which are areas of demyelination within the brain that cause dysfunction. Our research indicates another possibility: that bacterial RNA and protein within brain tissue lead to an immune response. That is, demyelination might just be the result of a normal immune response to microbial invasion. MS could be an unusual or slowly advancing infection that might resolve, progress, or stutter and relapse. The areas of demyelination are typically full of macrophages which are immune surveillance cells. These plaques also contain lymphocytes, neutrophils, antibodies, and complement. Thus, all components of the immune system are involved in the MS disease process, just like you would expect with an infection. What is missing is the actual cause of MS. Our team suspects that some of our bacterial candidates might fill that role, but more work needs to be performed. Q. Isn’t your microbial hypothesis of MS in conflict with the autoimmune concept of the disease? A. No, not really. There is no question that the beta-interferons and biologics have helped limit the number of MS relapses that patients experience. Multiple clinical trials proved that these interventions do help, and more aggressive immunosuppressive treatments (e.g steroids, rituximab) are often used off-label to control MS attacks. Some infections also require that the immune system response be controlled. For instance, patients with tuberculous meningitis, pneumococcal meningitis, and Pneumocystis pneumonia benefit from steroid use early on in the disease. We don’t really know at this stage of the research whether the microbial components within MS brain lesions are actively metabolizing or are just dead sequence and antigen. If the latter is true, controlling the immune response would be very important to limiting damage (i.e. demyelination) within the central nervous system (CNS). If the microbes we discovered are metabolically active, then specific drugs like carefully selected antibiotics might help, particularly in the earliest stages of the disease. Q. Where did the MS microbes you showed originate? Were they delivered to the brain through the bloodstream, or is some other mechanism suspected? A. There are at least two possibilities for the microbial sequence and antigen we found in MS brain lesions: 1) hematogenous seeding from the blood, or 2) delivery by the infiltrating macrophages. If bacteria are in fact seeded into MS lesions from the blood, it seems curious that this has not been discovered before. However, many of the MS microbial candidates identified in the study are anaerobic or even unculturable, and it is well established that recovery of microbes from brain abscesses (with a different pathologic appearance than demyelination) is often difficult. Macrophages could be bringing bacterial RNA and antigens into these MS lesions as a result of an autoimmune process. While macrophages and neutrophils are usually considered to be responders to tissue damage, not initiators, macrophages can actively participate in tissue destruction. In the end, the source of the microbial components may not matter very much. Adaptive immunity (i.e. antibody production) seems to be an important part of the MS disease process – hence the development of oligoclonal bands, demonstrating antibody synthesis within the CNS. It needs to be determined whether the MS microbial candidates we identified are driving a specific immune response or not. That is, are there antibodies in the spinal fluid against some of these MS candidate microbes? And, if so, do they account for some of the observed oligoclonal bands seen in most MS patients? These important questions, not answered by the present study, are topics for future investigation. Q. What can people do if they want to comment or get involved with the research? A. The journal Scientific Reports allows comments to accompany published papers. We encourage readers to leave a comment. We also welcome comments on our blog, and we suggest several ways to help promote MS research on our website.
CNS). The kidney or liver or bones can handle some scarring and retain normal function. The central nervous system is less resilient so the norm here for patients with MS is partial recovery of function or no recovery at all.
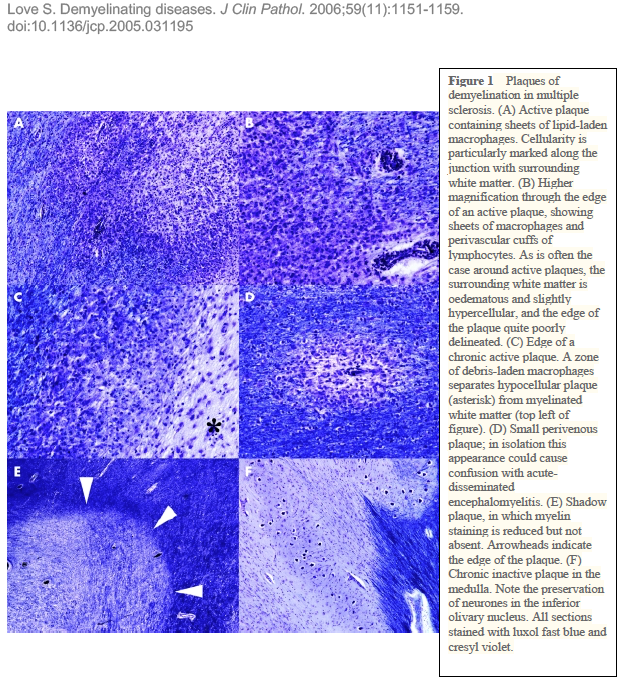
In a close up, what do these scarred areas look like? To quote Claudia Luchinetti, a Mayo Clinic neurologist, the pathologic hallmark of MS is the appearance of “seas of macrophages” within lesions. Lesions are areas damaged by disease. Macrophages are immune cells that are drawn into areas of damage. They fight to eliminate the threat and clean up the damage. The conventional thinking about MS holds that 1) the body’s innate autoimmune process (still largely a mystery), 2) causes the macrophages to come in and 3) damage the brain. Another possibility is that these many macrophages are just doing their job, killing the invaders, and that brain and spinal tissue get injured as “collateral damage.” I now think of MS as a train derailment, thanks to a leading Canadian professor who spoke at a recent MS meeting. Imagine that you are an alien with no preconceived notions about life on Earth, and you’re observing from your spaceship above. There is a train derailment and the train cars overturn and jump off the track. Passengers are hurt or killed. Within a short time, a “sea” of orange and yellow-jacketed responders arrives on the scene. They swarm the damaged and now stationary train, inside and out. But as an alien, you don’t really know if the orange jackets are doing good or evil. They could be finishing off the stricken passengers. Or, in the alternative, perhaps they are there to help. Of course, we Earthlings understand that the jacketed ones are first responders, stabilizing the situation, helping the injured, preventing further injuries and loss of life. But we know that only because we understand that a train derailment is an unusual and unwanted event. That is, we understand the cause of the problem, and we welcome its remedy. No one in their right mind would try to hinder the important work of the emergency workers. (Except perhaps a misguided alien with no concept of life on Earth.) This train derailment scenario sheds light on our scientific work with MS, where we don’t understand the cause but we can see the aftermath. That aftermath is an influx of lymphocytes and macrophages, effector cells of the immune system. Their work results in “demyelination” where myelin is lost, and the insulation on the wires of our nerve cells (axons) vanishes, causing microscopic short circuits. These short circuits lead to neurologic dysfunction and sometimes other debilitating symptoms. We do know this: The symptoms depend on the anatomic location of the train wreck. The field of MS study embraces so many questions: What are the inciting events that lead to demyelination? Are the infiltrating macrophages and lymphocytes leading to the tissue damage or are they limiting it? And, while we’re at it, is demyelination the root cause of the disease or is the cause the result of something else, like an infection or another autoimmune process? I, for one, want to crack the bad manners of MS. To truly understand the pathogenesis, I try to take a broad if not alien view of the disease. I know that demyelination is not exclusive to multiple sclerosis; other conditions trigger it as well. Influenza viruses occasionally get into the brain and cause widespread demyelination. The lowly JC virus also produces demyelination, usually in persons with a very impaired immune system (such as people with AIDS and the elderly). And demyelination of nerve cells does not stop at humans. The distemper virus that infects dogs causes demyelination and severe neurologic disease. These well-known examples of infections that lead to demyelination pull my view of MS way back. To me, as a researcher with three decades of experience, it makes sense to take a broad view, an inclusive view, and examine demyelination as an effect of something else. I believe that this enquiry will help us find both the cause and the remedy. Image credits: ID 68543427 © Pavlo Syvak | Dreamstime.com |
AuthorDr. John Kriesel is Associate Professor of Infectious Diseases at the University of Utah School of Medicine. He began this blog to raise awareness and generate discussion about the possible causes of multiple sclerosis. Archives
March 2021
Categories |




 RSS Feed
RSS Feed