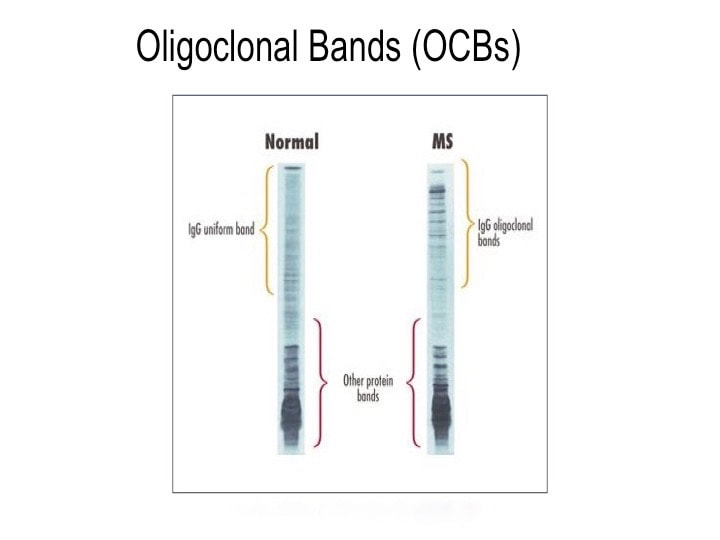
It includes proteins, electrolytes, antibodies, hormones, and sometimes outside agents like drugs and microorganisms. Doctors sometimes examine the antibodies present in patients’ serum to search for invading germs. And serology is a lousy way to diagnose disease. It’s inexact and not always timely. Antibodies are those little Y-shaped proteins made by the immune system (B-lymphocytes to be exact) that stick like glue to foreign invaders like viruses, bacteria, and other microbes. But they keep their cards close to their chests. For instance, Lyme disease takes weeks or even months to make antibodies against Borrelia burgdorferei, the bacterium that causes it. And infections with other bacteria can cause false positive tests for Lyme disease—even if those other infections occurred in the distant past. Call it “diagnostic archaeology,” because who really cares if you had Rocky Mountain spotted fever or some other infection years ago? You either died from it, or resolved it and live on. In multiple herpes cases, I have helped patients who were infected with herpes simplex virus (because I cultured it), yet their blood never made antibodies against the virus. Are those patients still infected with herpes or not? Actually, it’s not clear. You can see how how serologic diagnosis can lead to problems: over-diagnosis (false positives, as in Lyme testing) and under-diagnosis (false negatives, as in herpes testing). Sometimes serology tests are the best we can do. The syphilis agent, Treponema pallidum, has never been cultured in the lab. So if you want to figure out whether a patient has syphilis or not, you have to rely on serologies. And sometimes you just have to guess because patients with primary (the earliest form of) syphilis often don’t have a positive serologic because their immune system hasn’t had time to develop antibodies against the syphilis bacterium. Antibodies come in several forms. The most important is immunoglobulin G (IgG), which accounts for most antibodies in the blood and tissues. Other antibody types include IgA, which present in the gut and other mucosal surfaces, and IgE, responsible for many allergic responses. IgG immune responses to an infection are usually polyclonal, derived from many B-lymphocyte clones, each making slightly different antibodies, but all directed at one target. In contrast, B-lymphocyte cancers (myelomas) make only a single antibody species—a monoclonal. Oligoclonal (OCB) responses are neither monoclonal or polyclonal, but something in between with a few (oligo) B-lymphocytes producing a limited number of antibodies. OCBs are everywhere in MS patients’ spinal fluid and we have no idea why. Yet. Spinal fluid in healthy people contains very few antibodies—typically about 1/1000th the level in the blood. Past MS researchers tried to detect antibodies to a number of microbes, both in blood and spinal fluid. Wally Tourtellotte at UCLA led this effort, looking for antibodies in the spinal fluid of MS patients and comparing them to those found in the patients’ blood. To make a very long and complicated story short, his team basically found that MS patients are a little better at making antibodies compared to healthy control patients. But MS patients are not enough better at making antibodies to really account for their disease. And high levels of IgG antibody levels are often found in the spinal fluid of MS patients.
So what does spinal fluid serology have to do with MS? Everything, actually. Because “oligoclonal bands” are a terrible, confusing, and wordy way to describe IgG antibodies that collect in the spinal fluid. That’s right, most MS patients make IgG antibodies in their brain or spinal cord, and these collect in the spinal fluid. We can detect the presence of oligoclonal bands by a specialized test that uses an isoelectric focusing gel. Since all IgGs are about the same size, we use an electric charge to separate and visualize different antibody clones (also called species). These oligoclonal bands in the spinal fluid of MS patient are a hallmark of the disease – 90% of MS patients have them. When I was a medical student at Washington University in St. Louis in the 1980s, neurologist Charlie Trotter made big waves. He helped show that OCBs are commonly found in the spinal fluid of MS patients. At first, everyone believed these OCBs would be directed against myelin or some other human protein found in the brain, confirming the autoimmune nature of MS. Wrong! Sorry folks, these OCBs are not directed against myelin, myelin oligodendrocyte glycoprotein (MOG), or any other neural protein. In fact, 30+ years later no one really knows what these ever-present oligoclonal bands are doing. Oh sure, you can turn up the gain and show that some bands (antibody species) bind to Epstein Barr virus, or human herpes virus 6, but don’t believe that that’s the whole OCB enchilada. The actual targets of OCBs are still largely unknown. My lab team at the University of Utah intends to find out. Many MS researchers now believe that these OCBs are “poorly directed.” That is, they have no specific target but are produced haphazardly by the autoimmune process. I say these researchers are jumping to hasty conclusions. Because OCBs can also be found in the spinal fluid of patients with other diseases, particularly those involving neurologic infections. That is, patients with trypanosomiasis (African sleeping sickness), herpes simplex encephalitis, HIV, neurologic Lyme disease, neurologic syphilis, and neuroinvasive West Nile virus infection all have OCBs in their spinal fluid. It is logical that the targets of these OCBs are infecting or triggering organisms. It is predicted that the oligoclonal bands in specific MS patients are directed against microbes found in the brain, which our research has shown are often anaerobic bacteria (see the Microbes in MS sequencing paper recently published in Scientific Reports). MS patients need doctors and researchers to answer the biggest question: What are these tangled OCBs attacking in MS patients? Why are they there? What is their calling? Because once we know what the oligoclonal bands are aiming at, we can begin to intercede effectively. Said another way, the targets of these oligoclonal bands are the key to discovering the cause of MS. Royalty-free stock illustration ID: 81342454
0 Comments
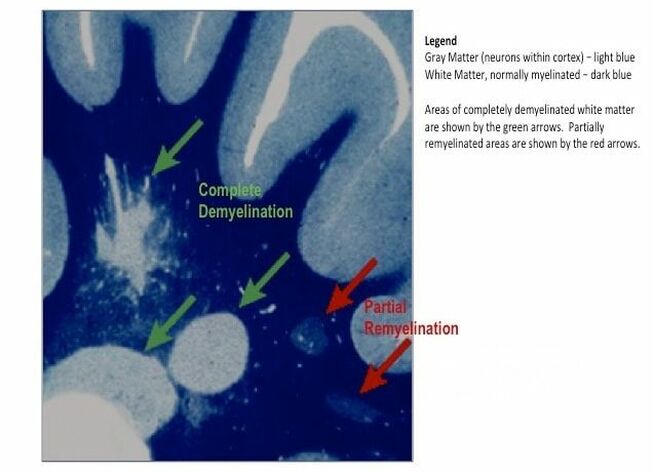
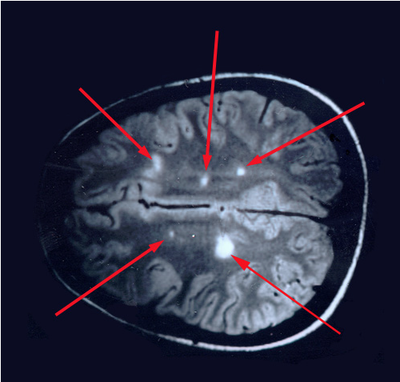
The journal Scientific Reports published the Microbes in MS team’s article titled “Spectrum of Microbial Sequences and a Bacterial Cell Wall Antigen in Primary Demyelination Brain Specimens Obtained from Living Patients” on February 4th. This Q & A reveals what the groundbreaking research paper contains. Q. What did you find? A. We discovered microbial (mostly bacterial) RNA sequences that are enriched in “plaques” (sites of diseased brain) taken from patients with multiple sclerosis (MS) compared to controls. One of the main findings from the study is that the MS samples were “dirtier” than the control samples. That is, on average, there were more RNA sequences mapping to microbes in the MS samples than in the controls. MS PlaquesAnother main finding of the study is that no single microbe is associated with the MS samples. That is, MS is not as simple as other neurologic infections (e.g. syphilis or herpes), but is much more complex. Most of our MS study subjects (10 of 11) had enriched sequences from several microbial taxa. There was some overlap, and the new paper specifically describes 29 MS Microbial Candidates. Surprisingly, many of these candidates are anaerobic, along with other kinds of bacteria. We suspect that these bacteria are playing a role in the disease process we call MS. Q. Why do RNA sequencing? What is so special about that? A. Sequencing allows us to see most or all RNA species in any given sample. That is, we can identify microbial sequence from microbes that are either 1) not viable due to the tissue processing or 2) unculturable altogether. Many such “unculturable” (or uncultivable) microbes exist; perhaps a majority of the microbial world has never been specifically isolated in culture. Another advantage of RNA sequencing, besides the microbial analysis, is that it allows us to look at human gene expression, too. Q. Do MS patients usually get brain biopsies? A. No, most MS patients never need a brain biopsy. However, occasionally there is some confusion about the diagnosis. Most of our subjects had a brain biopsy because their doctors suspected a malignancy (cancer). We were fortunate to find patients like these who had “primary demyelination” as their pathologic diagnosis. Most of these patients turned out to have MS in one form or another, although sometimes their doctors disagreed about the actual diagnosis. mri from a patient with msQ. Why did you see a need to run control specimens? Where did you get the controls? A. We felt that controls were very important because the MS brain specimens we studied are not sterile. That is, when these patients had their brain biopsies, the tissue was preserved in formalin, then embedded in paraffin wax in preparation for viewing under the microscope. While this process does preserve the architecture of the tissue and, fortunately, its RNA, it is not sterile. The control specimens were taken from patients with epilepsy who needed to have a piece of diseased brain removed to treat their seizures. The control brain tissue specimens were handled in the same way as the MS brain specimens. This allowed us to compare the microbial sequence content of each MS brain specimen with a set of 15 controls. This was done with each sample at more than 10,000 microbial taxa. Special statistical testing helped us draw some conclusions. Q. How do you know that these “MS Microbial Candidates” are meaningful? Couldn’t they just be an artifact of the preservation or sequencing process? A. We are not yet completely sure how meaningful these findings will be to the MS field. However, other groups have also shown a relationship between microbial components in the brain and the presence of MS. Couple this with the emerging view that microbes might play a role in normal functioning of the brain, and the team realized what a rich field of research this could be. We confirmed the presence of “peptidoglycan” – a component of bacterial cell walls – in several of our MS brain specimens. This leads us to believe that not only is microbial sequence (RNA) enriched in MS brain lesions, many of these lesions also contain bacterial protein (peptidoglycan). This could plausibly complete the link between microbes in MS brain tissue and the disease process. Q. What do you think about MS as an autoimmune disease? Is this view of the disease actually correct, or should we think about MS in some other way? A. The conventional view of MS is that it is “autoimmune,” where the human body is attacking itself, leading to “plaques,” which are areas of demyelination within the brain that cause dysfunction. Our research indicates another possibility: that bacterial RNA and protein within brain tissue lead to an immune response. That is, demyelination might just be the result of a normal immune response to microbial invasion. MS could be an unusual or slowly advancing infection that might resolve, progress, or stutter and relapse. The areas of demyelination are typically full of macrophages which are immune surveillance cells. These plaques also contain lymphocytes, neutrophils, antibodies, and complement. Thus, all components of the immune system are involved in the MS disease process, just like you would expect with an infection. What is missing is the actual cause of MS. Our team suspects that some of our bacterial candidates might fill that role, but more work needs to be performed. Q. Isn’t your microbial hypothesis of MS in conflict with the autoimmune concept of the disease? A. No, not really. There is no question that the beta-interferons and biologics have helped limit the number of MS relapses that patients experience. Multiple clinical trials proved that these interventions do help, and more aggressive immunosuppressive treatments (e.g steroids, rituximab) are often used off-label to control MS attacks. Some infections also require that the immune system response be controlled. For instance, patients with tuberculous meningitis, pneumococcal meningitis, and Pneumocystis pneumonia benefit from steroid use early on in the disease. We don’t really know at this stage of the research whether the microbial components within MS brain lesions are actively metabolizing or are just dead sequence and antigen. If the latter is true, controlling the immune response would be very important to limiting damage (i.e. demyelination) within the central nervous system (CNS). If the microbes we discovered are metabolically active, then specific drugs like carefully selected antibiotics might help, particularly in the earliest stages of the disease. Q. Where did the MS microbes you showed originate? Were they delivered to the brain through the bloodstream, or is some other mechanism suspected? A. There are at least two possibilities for the microbial sequence and antigen we found in MS brain lesions: 1) hematogenous seeding from the blood, or 2) delivery by the infiltrating macrophages. If bacteria are in fact seeded into MS lesions from the blood, it seems curious that this has not been discovered before. However, many of the MS microbial candidates identified in the study are anaerobic or even unculturable, and it is well established that recovery of microbes from brain abscesses (with a different pathologic appearance than demyelination) is often difficult. Macrophages could be bringing bacterial RNA and antigens into these MS lesions as a result of an autoimmune process. While macrophages and neutrophils are usually considered to be responders to tissue damage, not initiators, macrophages can actively participate in tissue destruction. In the end, the source of the microbial components may not matter very much. Adaptive immunity (i.e. antibody production) seems to be an important part of the MS disease process – hence the development of oligoclonal bands, demonstrating antibody synthesis within the CNS. It needs to be determined whether the MS microbial candidates we identified are driving a specific immune response or not. That is, are there antibodies in the spinal fluid against some of these MS candidate microbes? And, if so, do they account for some of the observed oligoclonal bands seen in most MS patients? These important questions, not answered by the present study, are topics for future investigation. Q. What can people do if they want to comment or get involved with the research? A. The journal Scientific Reports allows comments to accompany published papers. We encourage readers to leave a comment. We also welcome comments on our blog, and we suggest several ways to help promote MS research on our website. |
AuthorDr. John Kriesel is Associate Professor of Infectious Diseases at the University of Utah School of Medicine. He began this blog to raise awareness and generate discussion about the possible causes of multiple sclerosis. Archives
March 2021
Categories |
 RSS Feed
RSS Feed